 |
Member
|
|
Join Date: Oct 2009
Location: USA
Posts: 702
|
|
|
Member
Join Date: Oct 2009
Location: USA
Posts: 702
|
Thankyou and if it's ok to quote the article which I believe should:
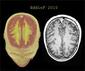
Patients receiving IFN alfa-n3 were significantly younger and had a longer disease duration than the other patients.
The MRI lesion loads and EDSS scores were significantly reduced at 1 year in the IFN alfa-n3 vs the untreated group.
Median lesion volume change was -7.2% for IFN beta-1b vs +11.7% for untreated patients and -10.4% for patients receiving IFN alfa-n3 (P = .009).
Mean EDSS change at 1 year was -0.20 in IFN beta-1b, +0.68 for untreated, and -1.13 for IFN alfa-n3 patients (IFN beta-1b vs untreated, P = .02; IFN alfa-n3 vs untreated, P = .0001).
No significant adverse effects were seen in the IFN alfa-n3 group, except for 1 case of deep venous thrombosis. These data should be interpreted with caution, since the study was open-label, retrospective, nonrandomized, and the clinical evaluations were unblinded.
However, further study of the efficacy of IFN alfa-n3 in MS is warranted. Dr. Sheremata, the senior author of the study, commented that "follow-up studies are planned" at his comprehensive MS center at the University of Miami.
Funny thing..my friend was in a Miami hospital..13? years ago.
I didn't see a article date. It performed a miracle on him. I'd like the same.
|